DOLORisk

DOLORisk: Understanding risk factors and determinants for neuropathic pain was a project on neuropathic pain, funded by the European Union, between 2015 and 2020. A consortium of European universities and small companies led by the University of Oxford studied the exact nature of risk factors for neuropathic pain and their interaction.
DOLORisk is a precursor to PAINSTORM. In PAINSTORM, we will expand the cohorts recruited in DOLORisk, by recruiting more participants and inviting participants for follow-up visits. This will allow us to study how neuropathic pain evolves over time. We will continue some of the work started in DOLORisk, for instance exploring genetic factors and building risk models for neuropathic pain.
What was DOLORisk about?
Neuropathic pain arises as a consequence of a disease or lesion in the somatosensory nervous system. Neuropathic pain is common, affecting 8% of the population, and will present a rising health burden in the future. It results in significant morbidity, reduces quality of life, and has a major deleterious impact on health in aging. However, not everyone with such a lesion develops significant neuropathic pain, and those who do develop it experience a wide range of severity, impact, and outcomes, and an unpredictable response to evidence-based treatment. This variation in pain prevalence and severity involves a complex interaction between genetics, environmental and clinical factors in a vulnerable individual.
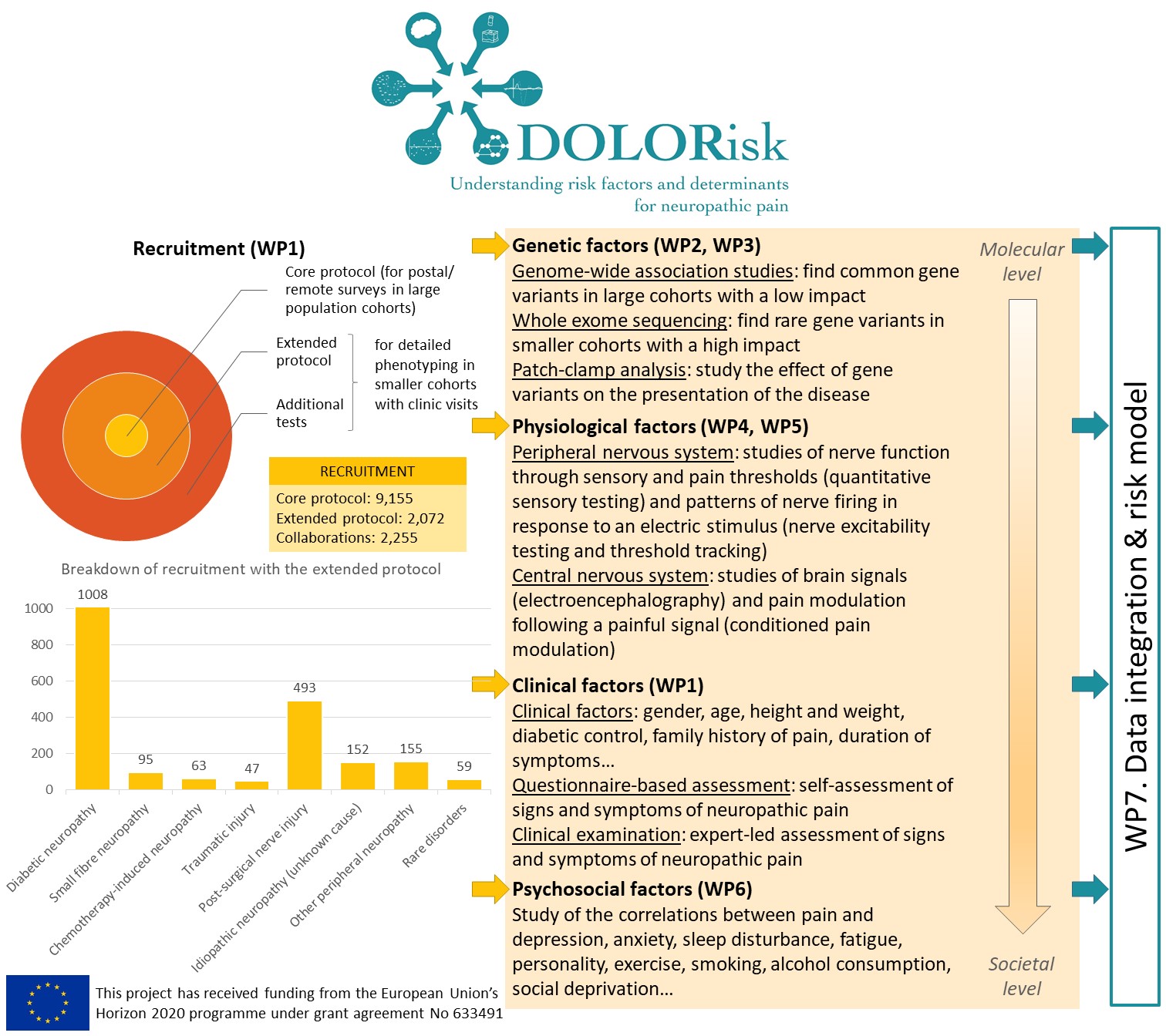
The nature of risk factors for neuropathic pain and their interaction were the focus of DOLORisk. We collected detailed clinical information and questionnaires on large cohorts of participants from which we identified genetic factors whose link with neuropathic pain had not been shown before. We studied the functional impact of ion channel variants and showed that these regulate nociceptor excitability. We found that conditioned pain modulation and electroencephalography were biomarkers that could distinguish between painful and painless neuropathy. However, other specialised tests like threshold tracking and quantitative sensory testing (QST)-derived sensory profile clusters did not show differences between the painful and painless groups. We investigated psychological risk factors and probed the validity of constructs commonly used in pain research. We used longitudinal community based cohorts to build algorithms to predict the development and maintenance of neuropathic pain. These emphasised the importance of psychological risk and resilience factors. These algorithms have been designed to be easily adaptable for clinical use and so can be validated in future independent longitudinal cohorts. We have therefore identified important risk factors for and pathophysiological mechanisms underlying neuropathic pain. The resulting dataset and the unprecedented number of biological samples we hold will be an essential resource for future studies of neuropathic pain.
What did it achieve?
The risk and impact of neuropathic pain represent a complex interplay of environmental and genetic factors which impinge on individual factors such as the emotional and cognitive state. The DOLORisk common protocol was defined to unpick these factors contributing to inter-individual variation. The size of the cohorts and the breadth and depth of our multidisciplinary approach ranging from molecular mechanisms to psychosocial factors have never been realised in the field of NP. With this approach we identified new mutations in sodium channels involved in painful diabetic neuropathy as well as other NP conditions such as non-freezing cold injury and rare conditions like congenital insensitivity to pain. These findings are already having impact as they have been integrated in clinical genetic testing algorithms. The human cellular models which we have validated are now being taken up by the pharmaceutical industry to facilitate analgesic drug development. Our GWAS studies have revealed new genes associated with neuropathic pain which can now be investigated by academics and industry in relation to drug discovery programmes.
In WP6 we probed the content of existing tools to predict chronic pain. In particular, we suggest that the construct of pain catastrophizing is not appropriate to understand the impact of pain on people living with chronic conditions. Instead, we found that pain catastrophizing overlaps largely the construct of pain-related worrying, which is a more neutral description of this psychological dimension of pain. This will aid assessment of the psychological correlates of chronic pain for research and clinical practice.
We developed a risk algorithm using the risk factors which we identified and tested this at a population level. This algorithm could have an important impact on prevention through identifying those at highest risk. A better understanding of the underlying pathophysiological mechanisms and furthermore patient stratification (according to genetic findings and sensory profile) will help improve clinical trial design and help predict which patients will respond to a given treatment. This will lead to more efficient pain management, personalised treatment, and a reduction in the social and economic consequences of living with chronic pain.
Further information
The following institutions were part of DOLORisk:
- University of Oxford (UK) (co-ordinator)
- Imperial College London (UK)
- University of Dundee (UK)
- Mentis Cura (Iceland)
- INSERM (France)
- Christian-Albrecht University Kiel (Germany)
- Lund University (Sweden)
- Technion - Israel Institute of Technology (Israel)
- Ghent University (Belgium)
- Neuroscience Technologies (Spain)
- Aarhus University (Denmark)
- Aarhus University Hospital (Denmark)
DOLORisk received funding from the European Union's Horizon 2020 funding programme, under grant agreement no. 633491.
DOLORisk outputs
- Rare Nav1.7 variants associated with painful diabetic peripheral neuropathy, DOI: 10.1097/j.pain.0000000000001116
- Defining the Functional Role of NaV1.7 in Human Nociception DOI: 10.1016/j.neuron.2019.01.047
- Cohort Profile: Genetics of Diabetes Audit and Research in Tayside Scotland (GoDARTS), DOI: 10.1093/ije/dyx140
- DOLORisk: study protocol for a multi-centre observational study to understand the risk factors and determinants of neuropathic pain, DOI: 10.12688/wellcomeopenres.14576.1
- Comprehensive analysis of long noncoding RNA expression in dorsal root ganglion reveals cell-type specificity and dysregulation after nerve injury, DOI: 10.1097/j.pain.0000000000001416
- Multidimensional screening for predicting pain problems in adults, DOI: 10.1097/pr9.0000000000000775
- Cold aggravates abnormal excitability of motor axons in oxaliplatin‐treated patients, DOI: 10.1002/mus.26852
- Let’s talk about pain catastrophizing measures: an item content analysis, DOI: 10.7717/peerj.8643
- Oxaliplatin‐ and docetaxel‐induced polyneuropathy: clinical and neurophysiological characteristics, DOI: 10.1111/jns.12413
- Cohort profile: DOLORisk Dundee: a longitudinal study of chronic neuropathic pain, DOI: 10.1136/bmjopen-2020-042887
- Systematic review and meta-analysis of genetic risk factors for neuropathic pain, DOI: 10.1097/j.pain.0000000000001164, Full text
- Association of Genetic Variant at Chromosome 12q23.1 With Neuropathic Pain Susceptibility, DOI: 10.1001/jamanetworkopen.2021.36560
- Classification of Painful or Painless Diabetic Peripheral Neuropathy and Identification of the Most Powerful Predictors Using Machine Learning Models in Large Cross-Sectional Cohorts, DOI: 10.1186/s12911-022-01890-x
- The characteristics of pain and dysesthesia in patients with diabetic polyneuropathy, DOI: 10.1371/journal.pone.0263831
- Conditioned pain modulation is more efficient in patients with painful diabetic polyneuropathy than those with nonpainful diabetic polyneuropathy, DOI: 10.1097/j.pain.0000000000002434
- Development and external validation of multivariable risk models to predict incident and resolved neuropathic pain: a DOLORisk Dundee study, DOI: 10.1007/s00415-022-11478-0
- Axonal Excitability Does Not Differ between Painful and Painless Diabetic or Chemotherapy-Induced Distal Symmetrical Polyneuropathy in a Multicenter Observational Study, DOI: 10.1002/ana.26319
- Electroencephalography functional connectivity—A biomarker for painful polyneuropathy, DOI: 10.1111/ene.15575
- Neuropathy and pain after breast cancer treatment: a prospective observational study, DOI: 10.1515/sjpain-2022-0017
- The epidemiology of neuropathic pain: an analysis of prevalence and associated factors in UK Biobank, DOI: 10.1101/2022.07.26.22278063
- Development and external validation of multivariable risk models to predict incident and resolved neuropathic pain: a DOLORisk Dundee study, DOI: 10.1007/s00415-022-11478-0
- A conceptual framework for causal effects within a biopsychosocial perspective of pain. DOI: 10.31219/osf.io/ug2mr
Other publications
- Neuropathic pain phenotyping by international consensus (NeuroPPIC) for genetic studies, DOI: 10.1097/j.pain.0000000000000335
- The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy, DOI: 10.1097/j.pain.0000000000000491
- Neuropathic pain: an updated grading system for research and clinical practice, DOI: 10.1097/j.pain.0000000000000492
- Reliability of conditioned pain modulation: a systematic review, DOI: 10.1097/j.pain.0000000000000689
- Risk factors for neuropathic pain in diabetes mellitus, DOI: 10.1097/j.pain.0000000000000785
- Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles, DOI: 10.1097/j.pain.0000000000000753
- Trigeminal neuralgia: New classification and diagnostic grading for practice and research, DOI: 10.1212/WNL.0000000000002840
- Neuropathic pain, DOI: 10.1038/nrdp.2017.2
- Stratifying patients with peripheral neuropathic pain based on sensory profiles, DOI: 10.1097/j.pain.0000000000000935
- A Genome-Wide Association Study Finds Genetic Associations with Broadly-Defined Headache in UK Biobank (N = 223,773), DOI: 10.1016/j.ebiom.2018.01.023
- A Genome-Wide Association Study of Diabetic Kidney Disease in Subjects With Type 2 Diabetes, DOI: 10.2337/db17-0914
- Neuropathic pain drives anxiety behavior in mice, results consistent with anxiety levels in diabetic neuropathy patients, DOI: 10.1097/PR9.0000000000000651
- A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy DOI: 10.1093/brain/awx337
- Using stratified medicine to understand, diagnose, and treat neuropathic pain DOI: 10.1097/j.pain.0000000000001301
- The novel activity of carbamazepine as an activation modulator extends from NaV1.7 mutations to the NaV1.8-S242T mutant channel from a patient with painful diabetic neuropathy DOI: 10.1124/mol.118.113076
- The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes DOI: 10.1016/s1474-4422(17)30329-0
- Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source DOI: 10.1093/brain/awx201
- New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain DOI: 10.1016/j.neuron.2017.02.005
- Axonal excitability changes and acute symptoms of oxaliplatin treatment: In vivo evidence for slowed sodium channel inactivation DOI: 10.1016/j.clinph.2017.11.015
- Germline selection shapes human mitochondrial DNA diversity DOI: 10.1126/science.aau6520
- Genome-wide association study of knee pain identifies associations with GDF5 and COL27A1 in UK Biobank DOI: 10.1038/s42003-019-0568-2
- The Genetics of Neuropathic Pain from Model Organisms to Clinical Application DOI: 10.1016/j.neuron.2019.09.018
- Whole-genome sequencing of patients with rare diseases in a national health system DOI: 10.1038/s41586-020-2434-2
- Neuropathic pain in the community: prevalence, impact, and risk factors DOI: 10.1097/j.pain.0000000000001824
- Long‐term symptoms of polyneuropathy in breast and colorectal cancer patients treated with and without adjuvant chemotherapy DOI: 10.1002/cam4.3129
- Axonal swellings are related to type 2 diabetes, but not to distal diabetic sensorimotor polyneuropathy DOI: 10.1007/s00125-020-05352-9
- Diabetic polyneuropathy and neuropathic pain: findings from a qualitative study DOI: 10.1002/pdi.2307
- Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management DOI: 10.1093/brain/awab079
- Genome-Wide Association Study of Peripheral Artery Disease DOI: 10.1161/circgen.119.002862
- Studying human nociceptors: from fundamentals to clinic DOI: 10.1093/brain/awab048
- Hepatocyte growth factor, colony-stimulating factor 1, CD40, and 11 other inflammation-related proteins are associated with pain in diabetic neuropathy: exploration and replication serum data from the Pain in Neuropathy Study DOI: 10.1097/j.pain.0000000000002451
- Acute small fiber neuropathy after Oxford-AstraZeneca ChAdOx1-S vaccination: A report of three cases and review of the literature DOI: 10.1111/jns.12509
- Investigating genotype–phenotype relationship of extreme neuropathic pain disorders in a UK national cohort, DOI: 10.1093/braincomms/fcad037